AFP
The US Justice Department urged an appeals court on Monday to freeze a ruling by a federal judge in Texas that would ban a widely used abortion pill in a far-reaching case that looks likely to wind up at the Supreme Court.
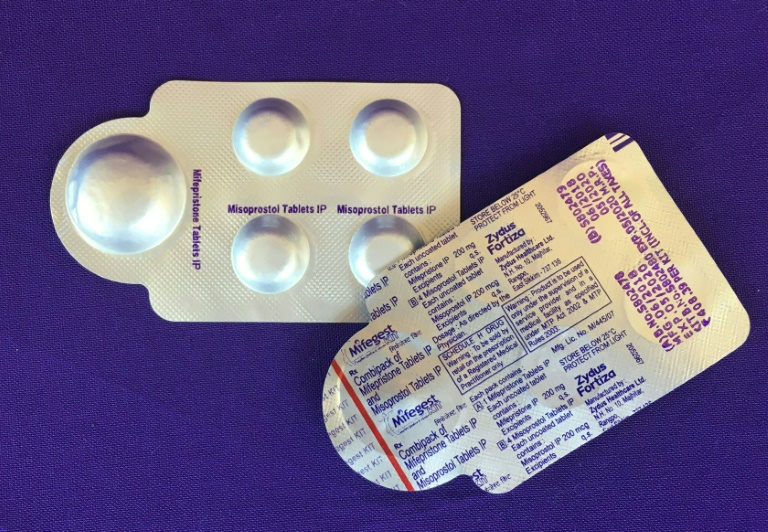
Judge Matthew Kacsmaryk, an appointee of former Republican president Donald Trump, on Friday overturned the Food and Drug Administration’s two-decade-old approval of mifepristone, which is used for more than half the abortions carried out annually in the United States.
“The district court’s extraordinary and unprecedented order should be stayed pending appeal,” the Justice Department said in a court filing.
“If allowed to take effect, the court’s order would thwart FDA’s scientific judgment and severely harm women,” the filing said. “This harm would be felt throughout the country, given that mifepristone has lawful uses in every state.”
The Justice Department asked the US Court of Appeals for the Fifth Circuit to stay the district judge’s order pending a full appeal in the case.
The latest US standoff over women’s reproductive rights comes almost a year after the conservative-dominated Supreme Court overturned the landmark Roe v. Wade ruling that enshrined a woman’s right to abortion for half a century.
President Joe Biden, a Democrat, pledged last week to fight the ruling that would ban mifepristone, calling it “an unprecedented step in taking away basic freedoms from women and putting their health at risk.”
“It is the next big step toward the national ban on abortion that Republican elected officials have vowed to make law in America,” Biden said.
White House Press Secretary Karine Jean-Pierre condemned the ruling on Monday as an “attack on FDA authority” and warned that it could “open the floodgates for other medications to be targeted and denied to people who need them.”
Shortly after the judge in Texas issued his decision, a judge in Washington state ruled in a separate case that access to mifepristone must be preserved.
District Judge Thomas Rice, a Barack Obama appointee, said mifepristone is “safe and legal” and the FDA must preserve access to it in more than a dozen states.
The dueling legal opinions, along with the appeals, means the issue is almost certain to end up before the Supreme Court.
Mifepristone is one component of a two-drug regimen that can be used in the United States through the first 10 weeks of pregnancy.
It has a long safety record, and the FDA estimates 5.6 million Americans have used it to terminate pregnancies since it was approved.
As the politically sensitive issue played out in the courts, the governors of states where abortion remains legal in the wake of last year’s Supreme Court ruling moved to protect access to medication abortion.
California Governor Gavin Newsom announced that his state has secured an emergency stockpile of up to two million pills of misoprostol, which is used in combination with mifepristone but can also be used on its own to induce an abortion.
“We will not cave to extremists who are trying to outlaw these critical abortion services,” Newsom said. “Medication abortion remains legal in California.”
In Massachusetts, Governor Maura Healey announced that the northeastern state had acquired 15,000 doses of mifepristone, a sufficient stockpile for a year.
More than 250 executives from leading pharmaceutical and biotech companies signed a letter meanwhile warning that a ruling by a judge with “no scientific training” undermines the drug approval authority of the FDA and “creates uncertainty for the entire biopharma industry.”
Judge Kacsmaryk’s ruling came after a coalition of anti-abortion groups sued to freeze the national distribution of mifepristone.
Kacsmaryk, in his decision, adopted language used by abortion opponents, referring to abortion providers as “abortionists” and saying the drug was used to “kill the unborn human.”
Kacsmaryk said the two-drug regimen that includes mifepristone had resulted in “thousands of adverse events suffered by women and girls,” including intense bleeding and psychological trauma.
But the FDA, researchers and the drugmaker say decades of experience have proven the medication to be safe and effective when used as indicated.
While he stayed the FDA’s 23-year-old approval, Kacsmaryk halted its enforcement for seven days to allow time for appeals.