While humans are living longer – a child born in 2021 on average could expect to live to 71 – we have not figured out how to beat one of the worst scourges of aging: Alzheimer’s disease.
Afflicting an estimated 36 million people worldwide, it is the most common form of dementia, a loss of cognitive functioning severe enough to interfere with daily life.
Researchers have yet to find a drug that reverses its progressive destruction of memory, thinking skills and, if sufferers live long enough, the ability to swallow and breathe.
Two new treatments – one that is available to patients and another nearing approval after a series of delays – have been shown to slow patients’ rate of decline. While the benefits are small and come with downsides, they have provided a rare burst of hope for the field of Alzheimer’s research.
What causes Alzheimer’s?
It is not clear. Researchers think the build-up of abnormal proteins seen in the brains of Alzheimer’s patients plays a central role.
One, called amyloid, forms clumps called plaques around brain cells. Their presence alone does not seem to trigger cognitive decline, given that some people with normal mental functioning have them. A second protein, tau, creates tangles within brain cells. And a third suspect, brain inflammation, is thought to contribute in some way, perhaps in an intermediary role.
GET BT IN YOUR INBOX DAILY
Start and end each day with the latest news stories and analyses delivered straight to your inbox.
Whatever the precise mechanisms, neurons – cells that send messages throughout the brain and the rest of the body – stop working over time and die.
What are the new treatments?
The drugs, both laboratory-made antibodies, are lecanemab from Eisai Co and Biogen – sold under the brand name Leqembi – and Eli Lilly & Co’s donanemab that is still under regulatory review.
On Monday (Jun 10), a panel of 11 US Food and Drug Administration (FDA) advisers unanimously backed donanemab, and the drug could be cleared this year. In trials, each of the drugs significantly reduced the brain amyloid levels of Alzheimer’s patients in the early stages of the disease.
More importantly, patients’ cognitive decline slowed. After 18 months, those on Leqembi had scores 27 per cent better than those given a placebo on a scale that measures six capacities – memory, orientation, problem solving, function inside and outside the home, and personal care.
In the donanemab trial, the figure was 29 per cent for all patients who got the drug, and 36 per cent for a subset with lower levels of tau protein.
Who can take these drugs?
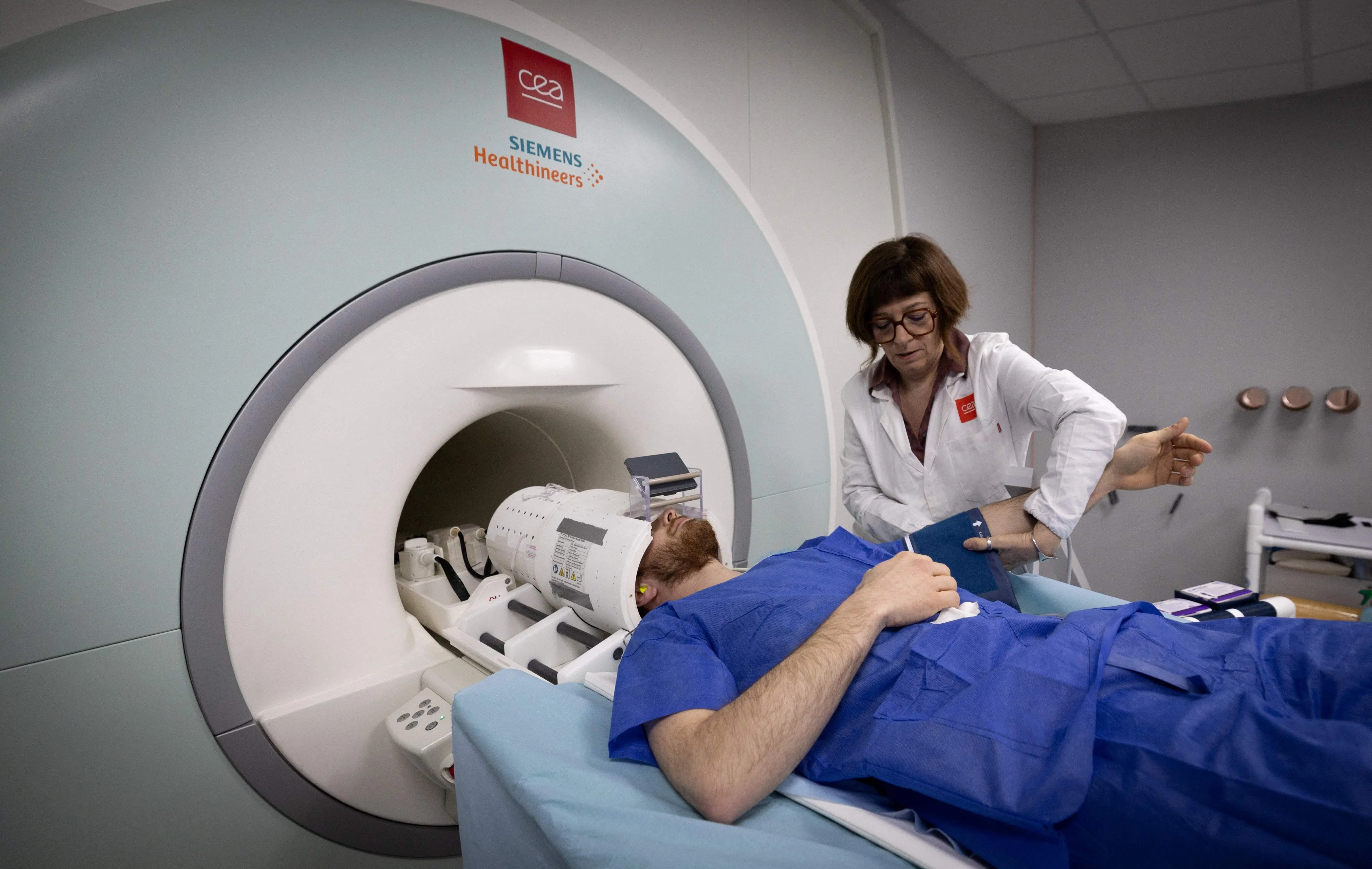
The FDA gave Leqembi full approval in July 2023 after clearing it on an expedited basis the previous January. The newer designation means that insurers will be more likely to cover the US$26,500 annual expense. It also came with restrictions, however. For example, MRI scans are required to monitor for early signs of side effects that can include brain bleeding and swelling.
Regulatory authorities have approved Leqembi in Japan and China, and its developers have filed for approval in the European Union. Leqembi and donanemab were proven effective only in people in the early stages of the disease, when cognition and functioning are mildly impaired. Their use is likely to be further limited by the need to be tested first for signs of brain amyloid.
An amyloid PET scan is one method, but it is expensive, often unavailable and, in the US, rarely covered by insurance. Another option is a spinal tap, in which a needle is inserted between two vertebrae to collect cerebrospinal fluid, but it is an invasive and sometimes painful procedure.
A few blood tests sold in the US can indicate whether amyloid is present in the brain, but none are routinely covered by insurers or cleared by the FDA. Japan’s health ministry approved such a test in late 2022.
What are the limitations of the drugs?
In the trials, the patients on them still declined, just somewhat more slowly than those who did not take them.
A significant drawback is that the drugs can cause brain swelling and bleeding. More than a fifth of people who took lecanemab experienced these effects and the figures were higher for donanemab. In most cases, these patients did not experience any related symptoms, but some had headaches, visual disturbances and confusion.
In the donanemab trial, three participants died after experiencing these side effects, while in the lecanemab study, five developed large brain hemorrhages. The trials enrolled between 1,700 and 1,800 people, split between groups that received the drug and a placebo.
The two drugs are administered as intravenous infusions – every two weeks for Leqembi and every four weeks for donanemab – which means patients must receive them at a health center. Leqembi is expensive, and Lilly has said it expects a similar price for donanemab.
Are there other Alzheimer’s drugs?
A handful of approved treatments modestly boost mental functioning temporarily in some patients, but they do not target the underlying problem: the destruction of neurons.
That was the aim of an earlier treatment from Biogen and Eisai, Aduhelm, which also was designed to reduce amyloid. It was approved by the FDA in 2021 despite conflicting trial results. The approval sparked controversy, and Aduhelm’s makers eventually stopped marketing the drug in the US.
What are the future paths for Alzheimer’s research?
The latest trial results strengthened the view that researchers are on the right track focusing on treatments that clear away amyloid. Still, it is not yet clear why lecanemab and donanemab succeeded where similar drugs failed.
Some researchers suspect that a certain threshold of amyloid removal must be met before a drug produces benefits. But with the pay-off limited even to those in the early phase of Alzheimer’s – possibly because damage to key brain areas is already extensive – some researchers are focused on getting ahead of the disease.
Brain scan studies suggest that amyloid starts accumulating as long as two decades before someone has dementia. In trials sponsored by Lilly and Eisai, researchers are testing amyloid-removing drugs on thousands of healthy adults. The hope is to stave off cognitive decline before it begins, or at least delay it.
Meanwhile, researchers are also developing drugs that target tau and brain inflammation. Bloomberg